Published online Jan 21, 2024. doi: 10.3748/wjg.v30.i3.211
Peer-review started: August 31, 2023
First decision: September 26, 2023
Revised: October 30, 2023
Accepted: December 14, 2023
Article in press: December 14, 2023
Published online: January 21, 2024
Colorectal cancer (CRC) screening is a fundamental tool in the prevention and early detection of one of the most prevalent and lethal cancers. Over the years, screening, particularly in those settings where it is well organized, has succeeded in reducing the incidence of colon and rectal cancer and improving the prognosis related to them. Despite considerable advancements in screening technologies and strategies, the effectiveness of CRC screening programs remains less than optimal. This paper examined the multifaceted reasons behind the persistent lack of effectiveness in CRC screening initiatives. Through a critical analysis of current methodologies, technological limitations, patient-related factors, and systemic challenges, we elucidated the complex interplay that hampers the successful reduction of CRC morbidity and mortality rates. While acknowledging the ad
Core Tip: Colorectal cancer (CRC) screening is a fundamental tool in the prevention and early detection of a prevalent and lethal cancers. Despite advancements in screening, the effectiveness of CRC screening programs remains less than optimal. This paper examined the multifaceted reasons behind the persistent lack of effectiveness in CRC screening initiatives. This study aimed to raise awareness of how CRC screening can reduce costs. Screening and early detection improve the prognosis of patients with CRC and result in an important reduction in the cost of treating advanced disease. Spending more sooner can mean saving money later.
- Citation: Tonini V, Zanni M. Why is early detection of colon cancer still not possible in 2023? World J Gastroenterol 2024; 30(3): 211-224
- URL: https://www.wjgnet.com/1007-9327/full/v30/i3/211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i3.211
From the latest reports of the National Cancer Institute, the number of “cancer survivors” is soaring, and projections are alarming. This phenomenon is due to the natural increase in population numbers, amplified by the lengthening of the average life span and to improved treatments that allow increasing survival for cancer patients.
As of January 2022, it was estimated that there were 18.1 million cancer survivors in the United States. This represents approximately 5.4% of the population. The number of cancer survivors is projected to increase by 24.4%, to 22.5 million, by 2032 and to 26.0 million by 2040. Over the next decade, the number of people who have lived 5 or more years after their cancer diagnosis is projected to increase approximately 30% to 16.3 million. Most (67%) survivors are currently age 65 or older. It is estimated that by 2040 74% of cancer survivors in the United States will be 65 or older.
In the light of these data, our first goal has been to treat patients with cancer and then to seek more effective and often more expensive treatments to achieve a patient’s cure or otherwise increase survival. However, if cancer survivors are increasing day by day, how are we going to take care of this growing volume of patients in need of treatment in the future? What strategies should we adopt to deal with this problem? Obviously, the first measure is undoubtedly to implement information campaigns on anti-cancer lifestyles and to put in place screening programs for early detection of the disease. It is intuitive that cancer costs less when diagnosed at an early stage, thus limiting expenses to surgery, length of stay, and follow-up. If it is diagnosed at a more advanced stage, costs will increase in an attempt to keep the disease under control for as long as possible.
The cancers we will have to address first will obviously be the most frequent ones, and among them is colorectal cancer (CRC). CRC is the third most common cancer in males and the second most common in females worldwide[1] and accounts for 10% of the total cancer burden[2]. Globally, nearly 2 million new cases of CRC (including anus) and more than 900000 deaths occur each year[3]. Incidence rates are approximately 4-fold higher in transitioned countries compared with transitioning countries, but there is less variation in the mortality rates because of higher fatality in transitioning countries[3]. The highest incidence rates of CRC are observed in European regions, Australia/New Zealand, and North America[3,4]. Lifetime risk of CRC is similar between females and males, 4.1% and 4.4%, respectively[5]. The dominant risk factor for CRC is age. Age-specific incidence and mortality increase dramatically over a lifetime, from 6 and 1 per 100000 people aged 30-34 years to 228 and 105 per 100000 people aged 80-84 years, respectively[5,6]. In 2021, Fang et al[7] performed an analysis of the clinical characteristics of CRC in the Chinese population (cohort of 13328 patients) and found that 58.1% of CRC cases were observed in individuals over 60-years-old. According to an even more recent study of the Chinese population, age > 65 years is a significant risk factor for developing CRC with an odds ratio of 1.4[8]. The 5-year survival rate for stage I colon cancer is 91% but drops to 72% for locally advanced disease and 14% for stage IV[4].
CRC occurs sporadically in 65%-70% of CRC cases, while the remaining 30%-35% of cases are genetic or familial forms, which should be recognized as early as possible and included in a close follow-up program. Polyps are considered precancerous lesions. About two-thirds of CRC cases develop through the adenomacarcinoma sequence, while the remaining one-third of CRC cases originate from the serrated pathway[9]. The neoplastic degeneration of a colorectal polyp to CRC occurs over a very long period, and we would therefore have a lot of time to recognize this polyp early and remove it before it becomes cancer. Resection of the polyp in CRC screening reduces the incidence and mortality of cancer[9,10]. In any case, we could still remove the cancer at a very early stage of the disease. Therefore, we are facing a disease that could be prevented by a simple endoscopy and instead bring an exaggerated number of new cases and deaths every year. Unfortunately, even in the most advanced countries and in those where screening programs are active, diagnosis is often late when the cancer is already in an advanced stage. As reported in the latest guidelines of the American Society of Colorectal Surgery[11], the diagnosis of CRC is made in 70% of cases when the patient is already symptomatic, often with symptoms such as hemorrhage or occlusion that require emergency surgery.
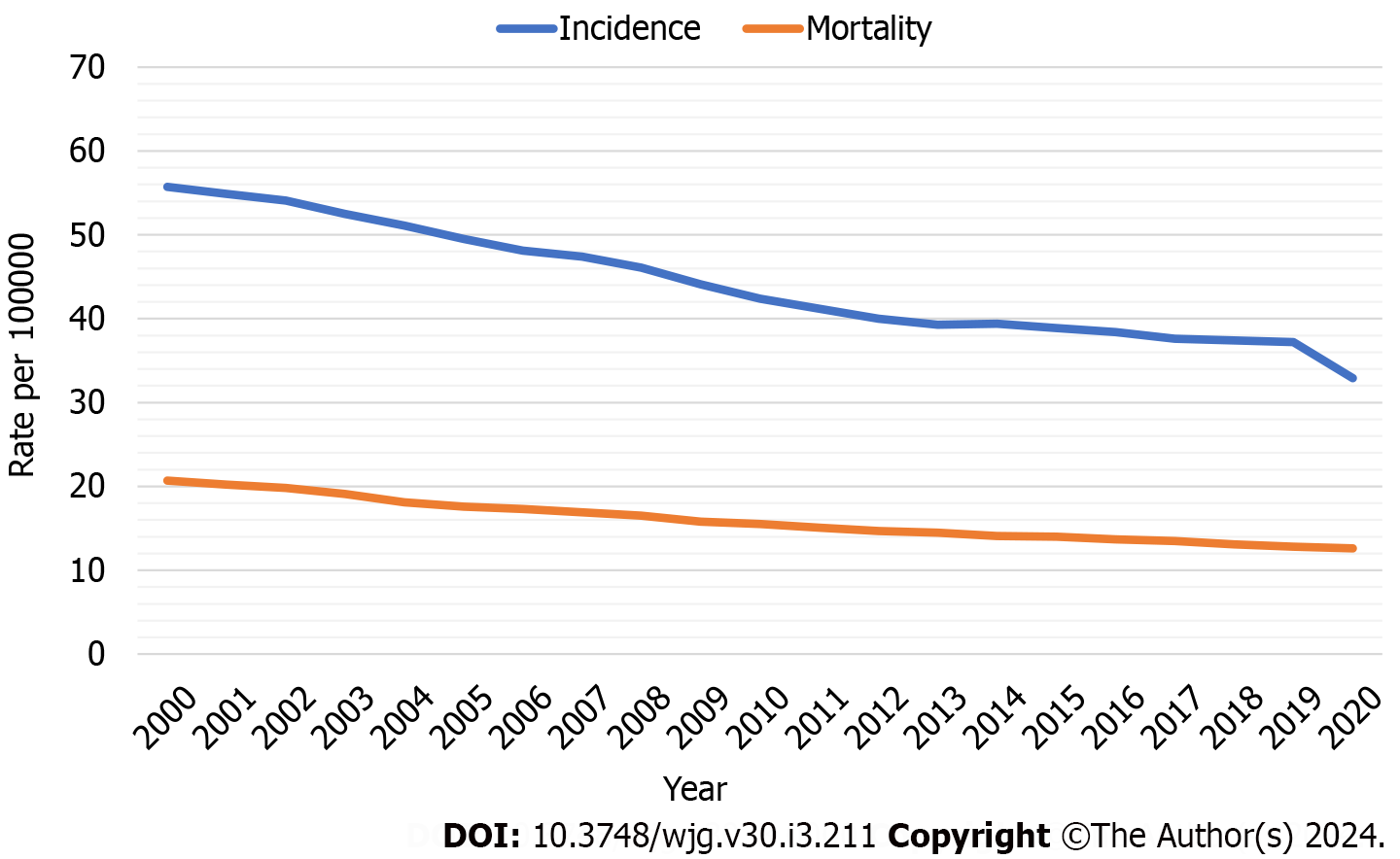
CRC incidence and mortality have declined over time (Figure 1) due to improvements in exposure to risk factors, treatment of diagnosed CRC, and widespread uptake of screening[5]. The observed trend correlates with an increase in the proportion of eligible individuals upgrading with screening[12]. From 2000 to 2018, CRC incidence and mortality decreased from 56 and 20 to 37 and 13 per 100000, respectively[13] while the proportion of individuals aged 50 years to 75 years who are up-to-date with screening increased from 34.6%[14] to 67.0%[15]. The benefit of colorectal screening in preventing specific deaths is between 25% and 50%[4,16].
The use of screening and the resulting early detection of more cases, in addition to having benefits in terms of survival and quality of life, could also have economic benefits[17]. In fact, despite a higher initial cost because the exams are performed on a large number of individuals considered healthy, early detection of CRC results in a global cost reduction. The positive cost impact in the lower cancer stages (stages I-II) can be explained by less invasive surgery, shorter hospital stays, fewer emergency admissions and outpatient visits within 12 mo of diagnosis, and less use of chemotherapy and biologic drugs. The potential cost savings associated with an early diagnosis are greater in patients age 18-64[18].
Gheysariyeha et al[19] conducted a systematic review with cost-effectiveness results showing that annual fecal immunochemical test (FIT), colonoscopy every 10 years, sigmoidoscopy every 5 years, and biennial high-sensitivity guaiac-based fecal occult blood test (HSgFOBT) and Stool DNA Test every 3 years were cost-saving strategies compared to not screening. In most of the studies, FIT in comparison with other strategies was cost-saving (less costly and more effective).
CRC is an ideal target for screening because it arises from precursors that take a long time (up to 10 years) to evolve into a malignancy, offering a window of opportunity for polypectomy and cancer prevention[20]. Current CRC screening methods are divided into invasive and noninvasive tests[21]. Noninvasive tests include stool-based tests, blood tests, and radiological examinations. Stool-based tests available are HSgFOBT, FIT, and fecal DNA test (Multitarget stool DNA, MT-sDNA, Cologuard®). Blood-based tests include Epi proColon, which detects circulating methylated SEPT9, and tests that detect microRNA and plasma protein biomarkers. Radiological examinations include computed tomographic colonoscopy (CTC) and capsule endoscopy (double-contrast barium enema is practically no longer applied in clinical practice)[21]. Invasive tests include flexible sigmoidoscopy and colonoscopy, which offer direct visualization and detection of a colon polyp or neoplasm with the advantage of obtaining a pathological specimen[21]. These are complemented by novel emerging screening modalities such as stool-based microbiome testing, urine-based screening tests using liquid chromatography-mass spectrometry or nuclear magnetic resonance spectroscopy, and magnetic resonance colonography. However, the novel tests are not optimal in terms of accuracy and depend on colonoscopy in case of abnormal results[22-24]. Currently, there are no data on whether the new screening strategies have an impact on CRC incidence and mortality, and they cannot, therefore, be recommended for CRC screening[22-24].
HSgFOBT detects colorectal polyps and cancers through an oxidation reaction of guaiaconic acid by hydrogen peroxide when the heme group is present in the stool sample. Sensitivity and specificity for CRC are 0.50-0.75 and 0.96-0.98[23], while for advanced adenomas are 0.06-0.17 and 0.96-0.99, respectively[23]. A 2019 meta-analysis showed that HSgFOBT screening led to a reduction in CRC-related mortality but did not reduce the incidence of CRC[25]. HSgFOBT has been largely replaced by FIT because it requires more samples, avoidance of red meat and drugs that can cause false positives, and because a positive test could be due to bleeding from anywhere in the gastrointestinal tract[5].
FIT is a screening test that detects the presence of the intact globin portion of human hemoglobin in stool using antibodies[26]. Considering the cutoff of 20 μgHb/g stool, the sensitivity and specificity for CRC are 0.74 and 0.94, respectively[23,24], while sensitivity and specificity for advanced adenoma are 0.23 and 0.96[21]. A 2015 study demonstrated a reduction in CRC mortality with biennial FIT but no change in CRC incidence[23].
Unlike HSgFOBT, FIT requires only a stool sample, is not influenced by the individual’s diet or medications, and does not present abnormal results in the presence of upper gastrointestinal bleeding because hemoglobin is partially digested before reaching the colon[26]. FIT is the most common noninvasive CRC screening modality among average-risk individuals. In a 2020 analysis, CRC detection rates were similar when four rounds of FIT in alternate years were compared with a single flexible sigmoidoscopy and a single colonoscopy[27]. An Italian intention-to-screen study evaluated the effectiveness of a 2-year screening program with FIT and found a stable 28% decrease in annual CRC incidence after 8 years[28].
The multi-target stool DNA testing (mt-sDNA screening test, also called Cologuard) is an Food and Drug Administration-approved noninvasive CRC screening tool. Cologuard uses a biomarker panel that analyzes a person’s stool sample for DNA markers as well as blood in the stool. The sensitivity and specificity for CRC are 0.93 and 0.85, respectively[23]. For advanced adenoma, the sensitivity is 0.43 and the specificity is 0.89[23,24]. With perfect adherence, mt-sDNA reduces the incidence of CRC by 66%[29]. Challenges of screening with mt-sDNA include cost and a high false-positive rate compared with FIT[29-31]. Overall, mt-sDNA is better than FIT in differentiating advanced precancerous lesions from non-neoplastic or negative findings[32]. However, its specificity is lower, which may result in more colonoscopies[31,22].
First described in the literature in 1994, CTC (also called CT colonoscopy, virtual colonography, and virtual colonoscopy) uses traditional computed tomography with image reconstruction techniques (3D rendering) to visualize the inner wall of the colon without the use of an endoscopic probe[33]. Sensitivity for adenomas 10 mm or greater is 0.89, and specificity is 0.94[23,24]. For adenomas 6 mm or larger, sensitivity is 0.86, and specificity is 0.88[23,24]. The advantages of CTC are less invasiveness, no need for procedural sedation, and low complication rate. Disadvantages are the need to prepare the bowel, exposure to radiation, the need to undergo colonoscopy in cases of positive results, and extracolonic findings involving further examination and potential overtreatment. The use of CTC is limited due to the lack of trained radiologists and imaging centers offering the test[24].
Colon capsule (CCE) is a noninvasive colon imaging technique involving the ingestion of a wireless pill-sized camera that takes images as it travels through the gastrointestinal tract. The first generation of CCE (PillCam-Colon) showed a sensitivity of 69% and specificity of 86% for detecting a polyp ≥ 6 mm in size[34]. The second generation of CCE (PillCam-Colon 2), which offers an adaptive frame rate and wider viewing angle, showed better accuracy in detecting polyps ≥ 6 mm in size, with a sensitivity of 84% and specificity of 88%[35]. It does not require air inflation, sedation, or the use of radiation and thus allows minimally invasive and painless colon evaluation. However, the rate of complete CCE examinations is only 67%[36], and 32% of CCEs result in referral to colonoscopy (polyps ≥ 10 mm)[37]. Interpretation of CCE also requires a physician skilled in reading capsule endoscopy and often takes longer than performing a colonoscopy[36]. The European Society for Gastrointestinal Endoscopy has proposed CCE as a screening tool in patients at average risk, in patients with incomplete colonoscopy, in patients who refuse conventional colonoscopy, and in patients with contraindications to conventional colonoscopy[38,39].
The detection of circulating and cell-free tumor DNA in blood has opened up the potential for blood-based tests for CRC and advanced malignancies, such as the search for SEPT9 DNA, C9orf50, KCNQ6, CLIP4, miRNA, interleukin-6, lectin serine protease 1 mannan binding, and integrin alpha 11[40-42]. Currently, only Epi proColon has been approved by the Food and Drug Administration as a blood-based screening test. Epi proColon detects circulating methylated SEPT9 DNA and has a sensitivity and specificity of 0.68 and 0.79 for CRC and 0.22 and 0.79 for advanced adenomas, respectively[43]. In general, a blood-based test is attractive because of its minimal invasiveness and the possibility of being combined with other routine tests. Adler et al[44] reported that 97% of people who refuse screening with colonoscopy accept a non
Colonoscopy is the most common screening modality in the United States and allows visual examination of the entire colon and rectum for polyps and CRC. Sensitivity is 0.89-0.95 and specificity is 0.89 for adenomas 10 mm or larger[23]. For CRC, the sensitivity is 0.18-1.00[23,24].
Cancer mortality is 29%-68% lower among people who undergo screening colonoscopy than those who do not[16,46-48]. The effectiveness of screening colonoscopy for CRC prevention was further quantified by a recent large randomized trial[49]. The 10-year risk of CRC was 0.98% among participants invited to undergo screening colonoscopy compared with 1.20% among those assigned to receive usual care. Screening colonoscopy was performed in only 42% of participants invited for screening. In analyses adjusted to estimate the effect of screening if all participants randomly assigned to screening actually underwent screening, the risk of CRC decreased from 1.22% to 0.84% (31% reduction) and the risk of death from CRC decreased from 0.30% to 0.15% (50% reduction)[49].
The disadvantages of colonoscopy are its invasiveness, risk of complications, need for bowel preparation, resource burden, and associated costs. Because of the financial and psychosocial barriers to adherence, colonoscopy is best reserved as the second stage of a two-stage screening cascade[50].
Flexible sigmoidoscopy is another option for direct visualization of the distal colon. Studies in the United Kingdom, Italy, and United States have reported a reduction in CRC incidence of 23% and 18%-23% and CRC mortality of 22%-31%[51-53]. However, due to the inability to evaluate the entire colon, the overall reduction in CRC incidence and CRC-related mortality is greater for colonoscopy than for flexible sigmoidoscopy[54]. The resources required for flexible sigmoidos
Although screening has had a positive effect on incidence and mortality, as previously reported, a significant percentage of CRC patients arrive at the hospital with urgent symptoms and advanced neoplasia[55]. About one-third of patients with CRC present as a surgical emergency[55].
Large bowel obstruction accounts for nearly 80% (15%-30% of CRCs) of CRC-related emergencies, while perforation accounts for the remaining 20% (1%-10% of CRCs)[56-59]. The most common site of CRC obstruction is the sigmoid colon, with 75% of tumors located distal to the splenic flexure[60]. Perforation occurs at the tumor site in almost 70% of cases and proximal to the tumor site in about 30% of cases[56,61]. Emergency surgery for CRC is associated with a worse prognosis than elective surgery, with lower overall and recurrence-free survival rates[59,62,63].
Such a high rate of urgent presentations of CRC should give pause to the still unsatisfactory results of screening. The ineffectiveness of early detection is due to the suboptimal accuracy of screening tools (particularly for polyps/adenomas), the poor adherence, the absence of screening programs in some areas of the world, the coronavirus disease 19 (COVID-19) pandemic, and the early onset of CRC.
Despite the various modalities offered for CRC screening, it is still underutilized. In the United States, screening rates remain around 60%[21,64]. Adherence to CRC screening is particularly poor among underserved populations, including low-income and African American and Hispanic populations. Over the past four decades, CRC incidence rates have decreased by 33.9% in United States Whites but only 6.6% in African Americans[2]. In 2015, 62.4% of males and females reported using a screening test for CRC[65]. Reported screening was lower among those aged 50-64 years (57.9%) than those aged 65-75 years (71.8%)[65]. The lowest use of screening for CRC was reported by people without a usual source of health care (26.3%) and uninsured people (25.1%)[65]. Adherence rates are no better in Asia-Pacific countries, ranging from 21.0% in South Korea to 62.9% in Thailand[66,67]. Participation rates ranging from 26% to 73% have been reported in Europe[68]. The European Union guidelines have proposed acceptable and desirable CRC screening adherence rates above 45% and 65%, respectively, and colonoscopy adherence among those with a positive primary screening test result above 90%[69,70]. The National Roundtable on CRC proposed an 80% adherence goal for primary screening, and the United States Multi-society Task Force on CRC set an 80% goal for colonoscopy adherence in patients with a positive FIT result[69,71,72]. Several factors play a role in influencing patient participation and sustained adherence. Barriers to screening include high costs, lack of adequate education about CRC, poor consideration of the benefits of screening, a sense of fatalism, or simply fear of screening tests[68,73].
The screening modality has an impact on the adherence rate. In general, the rule applies that more invasive tests have lower adherence rates[74]. In the COLONPREV randomized trial[75], patients underwent either colonoscopy or FIT, and the authors found participation rates of 25.0% and 34.2%, respectively. Similarly, in a meta-analysis comparing colonoscopy with CTC, the participation rates were 20.0% and 29.0%, respectively[68,76].
To achieve the highest level of adherence, it might be better to offer participants a choice because the “best” strategy is the one they will consistently adhere to[76]. Each step in effective CRC screening is associated with specific barriers. Each of these steps can occur in the opportunistic health care setting, such as independent private practices or individual hospitals. However, there are data demonstrating that implementation of programmatic or organized screening can result in improved adherence with CRC screening and benefits for outcomes[77]. An organized screening program is defined by the following characteristics: (1) An explicit policy with specified screening methods and intervals; (2) A defined target population; (3) A management team responsible for implementation; (4) A healthcare team for decision-making and assistance; (5) A quality assurance structure; and (6) A method for identifying cancer occurrence in the population[77,78]. Organized screening programs use a variety of evidence-based approaches to improve CRC screening uptake by members of the target population. These include sending patients invitations from their primary care provider, sending reminder letters, phone calls, sending fecal occult blood test/FIT kits to patients’ homes, and population-based public awareness campaigns[79-83]. Combinations of interventions have been associated with greater increases than single components[31
In a randomized trial, Libby et al[84] compared the rate of HSgFOBT adherence in 3 groups: invitation letter alone; invitation letter plus a prewarning letter; and the former two plus a CRC and screening information booklet. HSgFOBT uptake was highest in the group that received all three mailings. At the provider level, a recommendation to be screened from a primary care provider/general practitioner (GP) is clearly effective in raising participation[31]. Providing GPs with a list of their patients who were noncompliant with CRC screening resulted in a small increase in FIT screening at 1 year[85]. Boguradzka et al[86] found a higher participation rate for patients who received GP counseling on CRC screening than for those who received an information pamphlet (47.0% vs 13.7%).
Organized screening can reduce structural and economic barriers by expanding schedules, combining screening with other visits, such as the flu vaccination clinic, and making screening more convenient by offering passes or expanding insurance coverage[77]. Muliira and D’Souza[87] found improved participation rates from 11% to 91% with a patient navigator. Navigators were more effective in patients from minority groups. Selby et al[88] reported an adherence rate to diagnostic colonoscopy by FIT-positive subjects of more than 83% due to a combination of strategies, including insurance coverage that defines this procedure as preventive and telephone contact to schedule colonoscopy directly[87]. Eliminating economic barriers resulted in a substantial increase (ranging from 7% to 50%, depending on background rates of use) in population coverage, in particular among the low-income, least-educated subjects[68,89,90]
Organized screening programs can continuously monitor screening performance and clinical outcomes[91] and design interventions to address gaps. There are numerous examples of quality assurance programs related to the performance of colonoscopy, based on training and accreditation of endoscopy services[92-96]. Kaminski et al[94] tested a program to train endoscopy managers at low-performing facilities. They demonstrated improvements in the adenoma detection rate of the trained operator and the facility as a whole. In addition, they have shown that improved adenoma detection rates are associated with a decreased risk of interval cancer and cancer death[95,96].
Screening programs have reduced incidence, mortality, and surgery for CRC at the population level, but screening rates remain low in several countries[68,97,98]. Most screening in the United States occurs in the opportunistic setting. Organized CRC screening is more common in Europe than in the United States[97]. Opportunistic screening currently occurs in Latvia[99], Greece[99], and Bosnia-Herzegovina[100], while information on screening is lacking in Belarus, Slovakia, Liechtenstein, and Romania[98].
Similarly, most countries in Africa, Central America, South America, and the Middle East do not have organized screening programs[67], mainly due to the limited number of resources and the type of health system organization. Currently, organized screening is recommended in regions with the highest incidence of CRC (> 30 per 100000)[67,101]. Programs target individuals at average risk, aged 50 years to 75 years, and preferably apply the FIT test. Several East Asian countries have organized screening programs in place, including Japan, Korea, China, Hong Kong, Taiwan, and Bangkok[98,102]. In Asia the management of CRC screening is even more complex, as additional challenges are added, such as the lack of awareness of the usefulness of screening by some governments, government reluctance allocate money for building relevant infrastructure, inadequate manpower (too few surgeons and endoscopists relative to the population), and the issue of ethnicity[103-105]. In the case of multiethnic countries such as Malaysia, the risk of CRC is very different among Chinese, Malaysians, and Indians[106,107], with the incidence per 100000 population higher among Chinese and lower among Indians[106]. Therefore, it is difficult to reach consensus on the implementation of a national screening program in these regions[103].
In the United States, CRC screening is primarily based on colonoscopy, while in Europe most countries screen through FIT[108]. In Europe, a positive FIT must be followed by a colonoscopy within 1 mo[108]. Zorzi et al[109] reported that a delay of 9 mo after a positive FIT was associated with worse outcomes in terms of CRC risk and CRC progression. The same conclusion was reached by Lee et al[110] using data from the Taiwan Nationwide Screening Program while considering a 6-mo delay for colonoscopy after a positive FIT[110]. The coronavirus disease 2019 (COVID-19) pandemic beginning in March 2020 has overwhelmed the global healthcare system capacity and impacted the management of patients with cancer and other chronic diseases[111-113]. In response to the pandemic and to prevent COVID-19 infections and the spread of the virus in hospitals, there were global policy decisions like lockdowns. There was also redistribution of both human and material resources in the hospital setting[114,115]. This resulted in a drastic reduction of all non-essential services. Non-emergency visits, screenings, and elective surgeries were cancelled[116].
CRC management was severely affected by the pandemic. CRC screening activity decreased by up to 85%-95%. Care delivery was disrupted, and after resumption of activities, patients often refused colonoscopy for fear of being exposed to severe acute respiratory syndrome coronavirus 2, while planning processes were hampered by the need for viral testing prior to the procedure[117]. Delays in screening and surveillance resulted in the progression of precursor lesions and detection of tumors at a more advanced stage[108].
Meijer et al[118] reported a reduction in patients with stage I and II CRC from 29.5% and 26.6% to 20.0% and 25.5%, respectively, after the onset of the COVID-19 pandemic. They also noted an increase in patients with stage III and IV from 22.2% and 19.0% to 26.8% and 26.2%, respectively[118]. These changes were attributed to delays in CRC screening and diagnosis caused by the COVID-19 pandemic[112].
As a result, the mode of presentation of malignancy was also affected by the pandemic and the reduction in screening practices. Shinkwin et al[119] reported an increase in emergency presentations from 28.6% to 36.0%. Estimates suggested that there would be approximately 10000 excess deaths from breast cancer and CRC in the United States alone due to pandemic-related treatment interruptions[120], while 18800 people in the United States may experience delays in CRC diagnosis[121]. Similarly, population data in the United Kingdom suggest an increase in preventable cancer deaths due to COVID-19, with up to 16.6% of deaths due to CRC in the 5 years after diagnosis[122].
While overall CRC incidence rates have remained stable or declined in many high-income countries, incidence of early-onset CRC (generally defined as CRC that is diagnosed in individuals younger than 50 years) has recently been increasing worldwide, especially in the United States, Europe, Canada, Australia, New Zealand as well as in some countries in Asia[123]. Although there is still little certainty, early-onset CRC appears to be associated with the westernization of lifestyle[124]. Among early onset-CRC, about 30% of patients have mutations that cause inherited cancer predisposition syndromes, and 20% have familial CRC.
The average annual percent changes in early-onset CRC incidence were 4.0% in New Zealand, 2.8% in Canada and Australia, and 2.2% in the United States during 2008-2012[125]. In the United States, the age-adjusted early-onset CRC incidence per 100000 people was 5.9 cases in 2000 and 8.4 cases in 2017. Increases in early-onset CRC have also been documented in most European countries. Early-onset CRC incidence (per 100000 people) increased from 0.8 to 2.3 cases in individuals aged 20-29 years during 1990-2016, from 2.8 to 6.4 cases in those aged 30-39 during 2006-2016, and from 15.5 to 19.2 cases in those aged 40-49 during 2005-2016[126,127]. The average annual percent changes in early-onset CRC incidence were 7.9% in individuals aged 20-29, 4.9% in those aged 30-39, and 1.6% in those aged 40-49 during 2004-2016[127]. Taken together, early-onset CRC now represents a significant cancer burden among younger adults.
The increase of early-onset CRC incidence in the United States was initially largely driven by rectal cancer[126]. Since 2012 early-onset CRC incidence has increased similarly for colon and rectum with the annual percent change of approximately 1.8%[12]. The rise in early-onset CRC incidence appeared more prominent for colon cancer than for rectal cancer in Europe[127]. Within the next decade, the incidence rates of colon and rectal cancer are estimated to increase by 90% and 124%, respectively, among adults aged 20-34 years and 27% and 46% for those aged 35-49 years[128].
Patients with early-onset CRC are more likely to have synchronous and metachronous lesions and generally show a more advanced stage of disease because of lack of screening, poor consideration of symptoms, and reluctance to seek medical attention delay diagnosis[129,130]. Early-onset CRCs more frequently exhibit unfavorable histopathologic features, such as poor differentiation, perineural invasion, venous invasion, and mucinous and/or signet cell morphology[131,132].
Current population-based screening strategies need to be adapted. Therefore, the Multi-Society Task Force on CRC has recommended starting screening at age 45 years[133]. Early-onset CRC presents a challenge because most young adults diagnosed with CRC have no obvious risk factors and are classified as medium risk by current algorithms. Furthermore, because age and family history of cancer remain the cornerstones of CRC screening and risk stratification algorithms, empirical data supporting the effectiveness of screening young adults are lacking[134]. In fact, most of the landmark studies on screening involve patients over 50 years of age. However, half of all patients with early-onset CRC are younger than 45-years-old. Therefore, lowering the screening age will provide little or no benefit to these patients[135].
Ladabaum[136] reported a very interesting analysis on early onset-CRC, participation rates, and costs. By advancing the age of CRC screening participation in the United States by 5 years, it is estimated that 29400 cases and 11100 deaths from CRC could be averted in the next 5 years, at an incremental cost of about $10 billion and requiring nearly 11 million additional colonoscopies[136]. In comparison, achieving the goal of 80% screening participation at age 50 and above has been estimated to avert two-and-a-half times as many CRC cases and three times as many CRC deaths at an incremental cost of about one-third and requiring 13% more colonoscopies. The author then poses a crucial question: can the new recommendation be introduced without compromising efforts to achieve high screening participation rates in older or higher-risk people and higher FIT follow-up rates[136]?
In conclusion, despite significant advancements in medical technology, increased public awareness, and robust efforts to implement CRC screening programs, it is evident that the effectiveness of such initiatives still falls short of their intended goals. We delved into the intricate web of challenges and limitations that contribute to the persistent ineffectiveness of current CRC screening methodologies. The multifaceted nature of CRC, its biological heterogeneity, and the dynamic progression of the disease pose substantial hurdles to early detection and prevention. The limitations in sensitivity and specificity of screening tests, coupled with factors such as patient compliance, societal disparities, and healthcare accessibility issues, create a complex landscape that undermines the potential benefits of CRC screening. Missed lesions, overdiagnosis, interval cancers, and the failure to effectively address serrated lesions are all facets of the overarching problem of inadequate sensitivity and specificity of current screening methods. The invasive nature of certain procedures, the associated risks, and the psychological and emotional factors that deter patient participation, the delay in screening processes brought about by COVID-19, and the growing importance of early diagnosis of CRC further compound the challenge.
However, amidst these challenges, there remains room for optimism. Scientific research continues to advance our understanding of the intricate mechanisms underlying CRC, leading to the development of novel screening approaches and more personalized interventions. The integration of artificial intelligence, machine learning, and risk stratification models holds promise in refining screening algorithms and identifying high-risk populations that demand tailored approaches. Moreover, collaborations between medical professionals, researchers, policymakers, and the public are fundamental to surmounting the existing barriers. Public health campaigns, culturally sensitive education, and improved patient-physician communication have the potential to bolster compliance and participation rates. In the quest to enhance the effectiveness of CRC screening, it is crucial to acknowledge that there is no one-size-fits-all solution. A multifaceted strategy encompassing technological innovation, targeted interventions, policy changes, and patient empowerment is imperative. Only through persistent dedication to research, education, and patient-centered care can the medical community hope to meaningfully impact the trajectory of CRC and ultimately save lives.
Screening and early diagnosis not only reduce mortality and improve patient prognosis but also reduces health care costs. The positive cost impact in the lower cancer stages (stages I-II) can be explained by less invasive surgery, shorter hospital stays, fewer emergency admissions and outpatient visits, and less use of chemotherapy and biologic drugs. On the other hand, in patients with advanced CRC disease, we have to consider the costs of surgical reinterventions for recurrence or distant metastases, the high-cost drugs such as monoclonal antibodies and immunotherapy, the costs of radiotherapy, radiofrequency, transarterial chemoembolization, and all the techniques used to control a disease that has gotten out of control. However, we also have to consider the costs of absences from work for the patient and family members, costs of caregivers, colostomy supplies, home care and hospice admissions, etc. Clearly, spending more before results in a significant cost reduction afterwards.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qi L, China; Zhu L, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhao S
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18694] [Cited by in F6Publishing: 20880] [Article Influence: 2320.0] [Reference Citation Analysis (2)] |
| 2. | Bresalier RS. Colorectal Cancer Screening in a Changing World. Gastroenterol Clin North Am. 2022;51:577-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50630] [Cited by in F6Publishing: 44989] [Article Influence: 14996.3] [Reference Citation Analysis (47)] |
| 4. | Chetroiu D, Pop CS, Filip PV, Beuran M. How and why do we screen for colorectal cancer? J Med Life. 2021;14:462-467. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 5. | Gupta S. Screening for Colorectal Cancer. Hematol Oncol Clin North Am. 2022;36:393-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1570] [Cited by in F6Publishing: 2145] [Article Influence: 429.0] [Reference Citation Analysis (2)] |
| 7. | Fang L, Yang Z, Zhang M, Meng M, Feng J, Chen C. Clinical characteristics and survival analysis of colorectal cancer in China: a retrospective cohort study with 13,328 patients from southern China. Gastroenterol Rep (Oxf). 2021;9:571-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Liang L, Liang Y, Li K, Qin P, Lin G, Li Y, Xu H, Wang S, Jing Q, Liang B, Xu L. A risk-prediction score for colorectal lesions on 12,628 participants at high risk of colorectal cancer. Gastroenterol Rep (Oxf). 2022;10:goac002. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 9. | Chi Z, Lin Y, Huang J, Lv MY, Chen J, Chen X, Zhang B, Chen Y, Hu J, He X, Lan P. Risk factors for recurrence of colorectal conventional adenoma and serrated polyp. Gastroenterol Rep (Oxf). 2022;10:goab038. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Wu Y, Jiao N, Zhu R, Zhang Y, Wu D, Wang AJ, Fang S, Tao L, Li Y, Cheng S, He X, Lan P, Tian C, Liu NN, Zhu L. Identification of microbial markers across populations in early detection of colorectal cancer. Nat Commun. 2021;12:3063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 11. | Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, Thorsen AJ, Weiser MR, Chang GJ, Lightner AL, Feingold DL, Paquette IM. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis Colon Rectum. 2022;65:148-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 87] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 12. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2268] [Cited by in F6Publishing: 2793] [Article Influence: 698.3] [Reference Citation Analysis (2)] |
| 13. | Colon and Rectum Recent Trends in SEER Incidence and U.S. Mortality Rates, 2000–2018. In: SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance, Epidemiology, and End Results Program, National Cancer Institute. [Accessed August 27, 2021]. Available from: https://seer.cancer.gov/explorer/application.html?site=20&data_type=9&graph_type=2&compareBy=rate_type&chk_rate_type_2=2&chk_rate_type_3=3&sex=1&race=1&age_range=1&hdn_stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=1. [Cited in This Article: ] |
| 14. | Subramanian S, Amonkar MM, Hunt TL. Use of colonoscopy for colorectal cancer screening: evidence from the 2000 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2005;14:409-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | . QuickStats: Percentage of Adults Aged 50-75 Years Who Met Colorectal Cancer (CRC) Screening Recommendations*(,†) - National Health Interview Survey, United States, 2018(§). MMWR Morb Mortal Wkly Rep. 2020;69:314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 501] [Cited by in F6Publishing: 538] [Article Influence: 53.8] [Reference Citation Analysis (2)] |
| 17. | Khalili F, Najafi B, Mansour-Ghanaei F, Yousefi M, Abdollahzad H, Motlagh A. Cost-Effectiveness Analysis of Colorectal Cancer Screening: A Systematic Review. Risk Manag Healthc Policy. 2020;13:1499-1512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Laudicella M, Walsh B, Burns E, Smith PC. Cost of care for cancer patients in England: evidence from population-based patient-level data. Br J Cancer. 2016;114:1286-1292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | Gheysariyeha F, Rahimi F, Tabesh E, Hemami MR, Adibi P, Rezayatmand R. Cost-effectiveness of colorectal cancer screening strategies: A systematic review. Eur J Cancer Care (Engl). 2022;31:e13673. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 20. | Øines M, Helsingen LM, Bretthauer M, Emilsson L. Epidemiology and risk factors of colorectal polyps. Best Pract Res Clin Gastroenterol. 2017;31:419-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol. 2017;23:5086-5096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 332] [Cited by in F6Publishing: 333] [Article Influence: 47.6] [Reference Citation Analysis (9)] |
| 22. | Bosch LJW, Melotte V, Mongera S, Daenen KLJ, Coupé VMH, van Turenhout ST, Stoop EM, de Wijkerslooth TR, Mulder CJJ, Rausch C, Kuipers EJ, Dekker E, Domanico MJ, Lidgard GP, Berger BM, van Engeland M, Carvalho B, Meijer GA. Multitarget Stool DNA Test Performance in an Average-Risk Colorectal Cancer Screening Population. Am J Gastroenterol. 2019;114:1909-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325:1978-1998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 176] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 24. | Jain S, Maque J, Galoosian A, Osuna-Garcia A, May FP. Optimal Strategies for Colorectal Cancer Screening. Curr Treat Options Oncol. 2022;23:474-493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Jodal HC, Helsingen LM, Anderson JC, Lytvyn L, Vandvik PO, Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ Open. 2019;9:e032773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, Etzioni R, McKenna MT, Oeffinger KC, Shih YT, Walter LC, Andrews KS, Brawley OW, Brooks D, Fedewa SA, Manassaram-Baptiste D, Siegel RL, Wender RC, Smith RA. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250-281. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 945] [Cited by in F6Publishing: 1109] [Article Influence: 184.8] [Reference Citation Analysis (0)] |
| 27. | Grobbee EJ, van der Vlugt M, van Vuuren AJ, Stroobants AK, Mallant-Hent RC, Lansdorp-Vogelaar I, Bossuyt PMM, Kuipers EJ, Dekker E, Spaander MCW. Diagnostic Yield of One-Time Colonoscopy vs One-Time Flexible Sigmoidoscopy vs Multiple Rounds of Mailed Fecal Immunohistochemical Tests in Colorectal Cancer Screening. Clin Gastroenterol Hepatol. 2020;18:667-675.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Bucchi L, Mancini S, Baldacchini F, Ravaioli A, Giuliani O, Vattiato R, Zamagni F, Giorgi Rossi P, Campari C, Canuti D, Di Felice E, Sassoli de Bianchi P, Ferretti S, Bertozzi N, Biggeri A, Falcini F; Emilia-Romagna Region Workgroup for Colorectal Screening Evaluation. How a faecal immunochemical test screening programme changes annual colorectal cancer incidence rates: an Italian intention-to-screen study. Br J Cancer. 2022;127:541-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Redwood DG, Dinh TA, Kisiel JB, Borah BJ, Moriarty JP, Provost EM, Sacco FD, Tiesinga JJ, Ahlquist DA. Cost-Effectiveness of Multitarget Stool DNA Testing vs Colonoscopy or Fecal Immunochemical Testing for Colorectal Cancer Screening in Alaska Native People. Mayo Clin Proc. 2021;96:1203-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, Berger BM. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287-1297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1015] [Cited by in F6Publishing: 1064] [Article Influence: 106.4] [Reference Citation Analysis (1)] |
| 31. | Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 296] [Article Influence: 98.7] [Reference Citation Analysis (0)] |
| 32. | Shaukat A, Levin TR. Current and future colorectal cancer screening strategies. Nat Rev Gastroenterol Hepatol. 2022;19:521-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 86] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 33. | Philip AK, Lubner MG, Harms B. Computed tomographic colonography. Surg Clin North Am. 2011;91:127-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Rokkas T, Papaxoinis K, Triantafyllou K, Ladas SD. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest Endosc. 2010;71:792-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME, Carretero C, Charton JP, Van Gossum A, Wientjes CA, Sacher-Huvelin S, Delvaux M, Nemeth A, Petruzziello L, de Frias CP, Mayershofer R, Amininejad L, Dekker E, Galmiche JP, Frederic M, Johansson GW, Cesaro P, Costamagna G. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581-589.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 36. | Kroijer R, Kobaek-Larsen M, Qvist N, Knudsen T, Baatrup G. Colon capsule endoscopy for colonic surveillance. Colorectal Dis. 2019;21:532-537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Pecere S, Senore C, Hassan C, Riggi E, Segnan N, Pennazio M, Sprujievnik T, Rondonotti E, Baccarin A, Quintero E, Adrian de Ganzo Z, Costamagna G, Spada C. Accuracy of colon capsule endoscopy for advanced neoplasia. Gastrointest Endosc. 2020;91:406-414.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK, Benamouzig R, de Franchis R, Delvaux M, Devière J, Eliakim R, Fraser C, Hagenmuller F, Herrerias JM, Keuchel M, Macrae F, Munoz-Navas M, Ponchon T, Quintero E, Riccioni ME, Rondonotti E, Marmo R, Sung JJ, Tajiri H, Toth E, Triantafyllou K, Van Gossum A, Costamagna G; European Society of Gastrointestinal Endoscopy. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 39. | Han YM, Im JP. Colon Capsule Endoscopy: Where Are We and Where Are We Going. Clin Endosc. 2016;49:449-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Jensen SØ, Øgaard N, Ørntoft MW, Rasmussen MH, Bramsen JB, Kristensen H, Mouritzen P, Madsen MR, Madsen AH, Sunesen KG, Iversen LH, Laurberg S, Christensen IJ, Nielsen HJ, Andersen CL. Novel DNA methylation biomarkers show high sensitivity and specificity for blood-based detection of colorectal cancer-a clinical biomarker discovery and validation study. Clin Epigenetics. 2019;11:158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 41. | Bhardwaj M, Weigl K, Tikk K, Benner A, Schrotz-King P, Brenner H. Multiplex screening of 275 plasma protein biomarkers to identify a signature for early detection of colorectal cancer. Mol Oncol. 2020;14:8-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Zanutto S, Ciniselli CM, Belfiore A, Lecchi M, Masci E, Delconte G, Primignani M, Tosetti G, Dal Fante M, Fazzini L, Airoldi A, Vangeli M, Turpini F, Rubis Passoni GG, Viaggi P, Arena M, Motta RIO, Cantù AM, Crosta C, De Roberto G, Iannuzzi F, Cassinotti A, Dall'Olio V, Tizzoni L, Sozzi G, Meroni E, Bisanti L, Pierotti MA, Verderio P, Gariboldi M. Plasma miRNA-based signatures in CRC screening programs. Int J Cancer. 2020;146:1164-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 43. | Potter NT, Hurban P, White MN, Whitlock KD, Lofton-Day CE, Tetzner R, Koenig T, Quigley NB, Weiss G. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem. 2014;60:1183-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 44. | Adler A, Geiger S, Keil A, Bias H, Schatz P, deVos T, Dhein J, Zimmermann M, Tauber R, Wiedenmann B. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol. 2014;14:183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 45. | Ferrari A, Neefs I, Hoeck S, Peeters M, Van Hal G. Towards Novel Non-Invasive Colorectal Cancer Screening Methods: A Comprehensive Review. Cancers (Basel). 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 46. | Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128-1137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 47. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1952] [Cited by in F6Publishing: 2085] [Article Influence: 173.8] [Reference Citation Analysis (1)] |
| 48. | Kahi CJ, Pohl H, Myers LJ, Mobarek D, Robertson DJ, Imperiale TF. Colonoscopy and Colorectal Cancer Mortality in the Veterans Affairs Health Care System: A Case-Control Study. Ann Intern Med. 2018;168:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 49. | Bretthauer M, Løberg M, Wieszczy P, Kalager M, Emilsson L, Garborg K, Rupinski M, Dekker E, Spaander M, Bugajski M, Holme Ø, Zauber AG, Pilonis ND, Mroz A, Kuipers EJ, Shi J, Hernán MA, Adami HO, Regula J, Hoff G, Kaminski MF; NordICC Study Group. Effect of Colonoscopy Screening on Risks of Colorectal Cancer and Related Death. N Engl J Med. 2022;387:1547-1556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 183] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 50. | Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, Perdue LA, Lin JS, Siegel RL, Doria-Rose VP, Feuer EJ, Zauber AG, Kuntz KM, Lansdorp-Vogelaar I. Colorectal Cancer Screening: An Updated Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325:1998-2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 51. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J; UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1121] [Cited by in F6Publishing: 1083] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 52. | Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C, Crosta C, Falcini F, Ferrero F, Giacomin A, Giuliani O, Santarelli A, Visioli CB, Zanetti R, Atkin WS, Senore C; SCORE Working Group. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103:1310-1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 427] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 53. | Holme Ø, Løberg M, Kalager M, Bretthauer M, Hernán MA, Aas E, Eide TJ, Skovlund E, Schneede J, Tveit KM, Hoff G. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312:606-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 279] [Cited by in F6Publishing: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 54. | Ko CW, Doria-Rose VP, Barrett MJ, Kamineni A, Enewold L, Weiss NS. Screening colonoscopy and flexible sigmoidoscopy for reduction of colorectal cancer incidence: A case-control study. PLoS One. 2019;14:e0226027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Biondo S, Gálvez A, Ramírez E, Frago R, Kreisler E. Emergency surgery for obstructing and perforated colon cancer: patterns of recurrence and prognostic factors. Tech Coloproctol. 2019;23:1141-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Pisano M, Zorcolo L, Merli C, Cimbanassi S, Poiasina E, Ceresoli M, Agresta F, Allievi N, Bellanova G, Coccolini F, Coy C, Fugazzola P, Martinez CA, Montori G, Paolillo C, Penachim TJ, Pereira B, Reis T, Restivo A, Rezende-Neto J, Sartelli M, Valentino M, Abu-Zidan FM, Ashkenazi I, Bala M, Chiara O, De' Angelis N, Deidda S, De Simone B, Di Saverio S, Finotti E, Kenji I, Moore E, Wexner S, Biffl W, Coimbra R, Guttadauro A, Leppäniemi A, Maier R, Magnone S, Mefire AC, Peitzmann A, Sakakushev B, Sugrue M, Viale P, Weber D, Kashuk J, Fraga GP, Kluger I, Catena F, Ansaloni L. 2017 WSES guidelines on colon and rectal cancer emergencies: obstruction and perforation. World J Emerg Surg. 2018;13:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 57. | Gunnarsson H, Holm T, Ekholm A, Olsson LI. Emergency presentation of colon cancer is most frequent during summer. Colorectal Dis. 2011;13:663-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Barnett A, Cedar A, Siddiqui F, Herzig D, Fowlkes E, Thomas CR Jr. Colorectal cancer emergencies. J Gastrointest Cancer. 2013;44:132-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 59. | EuroSurg Collaborative. Acute PresentatiOn of coLorectaL cancer - an internatiOnal snapshot (APOLLO): Protocol for a prospective, multicentre cohort study. Colorectal Dis. 2023;25:144-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg. 2014;207:127-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 61. | Zielinski MD, Merchea A, Heller SF, You YN. Emergency management of perforated colon cancers: how aggressive should we be? J Gastrointest Surg. 2011;15:2232-2238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Cortet M, Grimault A, Cheynel N, Lepage C, Bouvier AM, Faivre J. Patterns of recurrence of obstructing colon cancers after surgery for cure: a population-based study. Colorectal Dis. 2013;15:1100-1106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P; collaborative Dutch Stent-In study group. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 64. | Sabatino SA, White MC, Thompson TD, Klabunde CN; Centers for Disease Control and Prevention (CDC). Cancer screening test use - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:464-468. [PubMed] [Cited in This Article: ] |
| 65. | White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, Geiger AM, Richardson LC. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:201-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 361] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 66. | Kew GS, Koh CJ. Strategies to Improve Persistent Adherence in Colorectal Cancer Screening. Gut Liver. 2020;14:546-552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632-3642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 361] [Cited by in F6Publishing: 329] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 68. | Dressler J, Johnsen AT, Madsen LJ, Rasmussen M, Jorgensen LN. Factors affecting patient adherence to publicly funded colorectal cancer screening programmes: a systematic review. Public Health. 2021;190:67-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | Kaminski MF, Robertson DJ, Senore C, Rex DK. Optimizing the Quality of Colorectal Cancer Screening Worldwide. Gastroenterology. 2020;158:404-417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 70. | European Colorectal Cancer Screening Guidelines Working Group; von Karsa L, Patnick J, Segnan N, Atkin W, Halloran S, Lansdorp-Vogelaar I, Malila N, Minozzi S, Moss S, Quirke P, Steele RJ, Vieth M, Aabakken L, Altenhofen L, Ancelle-Park R, Antoljak N, Anttila A, Armaroli P, Arrossi S, Austoker J, Banzi R, Bellisario C, Blom J, Brenner H, Bretthauer M, Camargo Cancela M, Costamagna G, Cuzick J, Dai M, Daniel J, Dekker E, Delicata N, Ducarroz S, Erfkamp H, Espinàs JA, Faivre J, Faulds Wood L, Flugelman A, Frkovic-Grazio S, Geller B, Giordano L, Grazzini G, Green J, Hamashima C, Herrmann C, Hewitson P, Hoff G, Holten I, Jover R, Kaminski MF, Kuipers EJ, Kurtinaitis J, Lambert R, Launoy G, Lee W, Leicester R, Leja M, Lieberman D, Lignini T, Lucas E, Lynge E, Mádai S, Marinho J, Maučec Zakotnik J, Minoli G, Monk C, Morais A, Muwonge R, Nadel M, Neamtiu L, Peris Tuser M, Pignone M, Pox C, Primic-Zakelj M, Psaila J, Rabeneck L, Ransohoff D, Rasmussen M, Regula J, Ren J, Rennert G, Rey J, Riddell RH, Risio M, Rodrigues V, Saito H, Sauvaget C, Scharpantgen A, Schmiegel W, Senore C, Siddiqi M, Sighoko D, Smith R, Smith S, Suchanek S, Suonio E, Tong W, Törnberg S, Van Cutsem E, Vignatelli L, Villain P, Voti L, Watanabe H, Watson J, Winawer S, Young G, Zaksas V, Zappa M, Valori R. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013;45:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 71. | Meester RG, Doubeni CA, Zauber AG, Goede SL, Levin TR, Corley DA, Jemal A, Lansdorp-Vogelaar I. Public health impact of achieving 80% colorectal cancer screening rates in the United States by 2018. Cancer. 2015;121:2281-2285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 72. | Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, Lieberman D, Levin TR, Rex DK. Recommendations on Fecal Immunochemical Testing to Screen for Colorectal Neoplasia: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;152:1217-1237.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 73. | Doubeni CA, Corley DA, Zauber AG. Colorectal Cancer Health Disparities and the Role of US Law and Health Policy. Gastroenterology. 2016;150:1052-1055. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 74. | Deibel A, Deng L, Cheng CY, Schlander M, Ran T, Lang B, Krupka N, Beerenwinkel N, Rogler G, Wiest R, Sonnenberg A, Poleszczuk J, Misselwitz B. Evaluating key characteristics of ideal colorectal cancer screening modalities: the microsimulation approach. Gastrointest Endosc. 2021;94:379-390.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Castells A, Quintero E. Programmatic screening for colorectal cancer: the COLONPREV study. Dig Dis Sci. 2015;60:672-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 76. | Duarte RB, Bernardo WM, Sakai CM, Silva GL, Guedes HG, Kuga R, Ide E, Ishida RK, Sakai P, de Moura EG. Computed tomography colonography versus colonoscopy for the diagnosis of colorectal cancer: a systematic review and meta-analysis. Ther Clin Risk Manag. 2018;14:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | Dominitz JA, Levin TR. What Is Organized Screening and What Is Its Value? Gastrointest Endosc Clin N Am. 2020;30:393-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | International Agency for Research on Cancer. Cervix cancer screening. IARC Handbook of Cancer Prevention, vol. 10. Lyon, France: IARC Press, 2005: 117–162. [Cited in This Article: ] |
| 79. | Baron RC, Rimer BK, Coates RJ, Kerner J, Kalra GP, Melillo S, Habarta N, Wilson KM, Chattopadhyay S, Leeks K; Task Force on Community Preventive Services. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35:S56-S66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 80. | Reuland DS, Brenner AT, Hoffman R, McWilliams A, Rhyne RL, Getrich C, Tapp H, Weaver MA, Callan D, Cubillos L, Urquieta de Hernandez B, Pignone MP. Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population: A Randomized Clinical Trial. JAMA Intern Med. 2017;177:967-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 81. | Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, Fuller S. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158:301-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 82. | Coronado GD, Petrik AF, Vollmer WM, Taplin SH, Keast EM, Fields S, Green BB. Effectiveness of a Mailed Colorectal Cancer Screening Outreach Program in Community Health Clinics: The STOP CRC Cluster Randomized Clinical Trial. JAMA Intern Med. 2018;178:1174-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 83. | Jager M, Demb J, Asghar A, Selby K, Mello EM, Heskett KM, Lieberman AJ, Geng Z, Bharti B, Singh S, Gupta S. Mailed Outreach Is Superior to Usual Care Alone for Colorectal Cancer Screening in the USA: A Systematic Review and Meta-analysis. Dig Dis Sci. 2019;64:2489-2496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 84. | Libby G, Bray J, Champion J, Brownlee LA, Birrell J, Gorman DR, Crighton EM, Fraser CG, Steele RJ. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen. 2011;18:24-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 85. | Rat C, Pogu C, Le Donné D, Latour C, Bianco G, Nanin F, Cowppli-Bony A, Gaultier A, Nguyen JM. Effect of Physician Notification Regarding Nonadherence to Colorectal Cancer Screening on Patient Participation in Fecal Immunochemical Test Cancer Screening: A Randomized Clinical Trial. JAMA. 2017;318:816-824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Boguradzka A, Wiszniewski M, Kaminski MF, Kraszewska E, Mazurczak-Pluta T, Rzewuska D, Ptasinski A, Regula J. The effect of primary care physician counseling on participation rate and use of sedation in colonoscopy-based colorectal cancer screening program--a randomized controlled study. Scand J Gastroenterol. 2014;49:878-884. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Muliira JK, D'Souza MS. Effectiveness of patient navigator interventions on uptake of colorectal cancer screening in primary care settings. Jpn J Nurs Sci. 2016;13:205-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 88. | Selby K, Jensen CD, Zhao WK, Lee JK, Slam A, Schottinger JE, Bacchetti P, Levin TR, Corley DA. Strategies to Improve Follow-up After Positive Fecal Immunochemical Tests in a Community-Based Setting: A Mixed-Methods Study. Clin Transl Gastroenterol. 2019;10:e00010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 89. | Fedewa SA, Goodman M, Flanders WD, Han X, Smith RA, M Ward E, Doubeni CA, Sauer AG, Jemal A. Elimination of cost-sharing and receipt of screening for colorectal and breast cancer. Cancer. 2015;121:3272-3280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 90. | de Moor JS, Cohen RA, Shapiro JA, Nadel MR, Sabatino SA, Robin Yabroff K, Fedewa S, Lee R, Paul Doria-Rose V, Altice C, Klabunde CN. Colorectal cancer screening in the United States: Trends from 2008 to 2015 and variation by health insurance coverage. Prev Med. 2018;112:199-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 91. | Rutter MD, Beintaris I, Valori R, Chiu HM, Corley DA, Cuatrecasas M, Dekker E, Forsberg A, Gore-Booth J, Haug U, Kaminski MF, Matsuda T, Meijer GA, Morris E, Plumb AA, Rabeneck L, Robertson DJ, Schoen RE, Singh H, Tinmouth J, Young GP, Sanduleanu S. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology. 2018;155:909-925.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 92. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1251] [Cited by in F6Publishing: 1384] [Article Influence: 138.4] [Reference Citation Analysis (0)] |
| 93. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1357] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 94. | Kaminski MF, Anderson J, Valori R, Kraszewska E, Rupinski M, Pachlewski J, Wronska E, Bretthauer M, Thomas-Gibson S, Kuipers EJ, Regula J. Leadership training to improve adenoma detection rate in screening colonoscopy: a randomised trial. Gut. 2016;65:616-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 95. | Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R, Rupinska M, Kocot B, Chaber-Ciopinska A, Pachlewski J, Polkowski M, Regula J. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 319] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 96. | Siau K, Green JT, Hawkes ND, Broughton R, Feeney M, Dunckley P, Barton JR, Stebbing J, Thomas-Gibson S. Impact of the Joint Advisory Group on Gastrointestinal Endoscopy (JAG) on endoscopy services in the UK and beyond. Frontline Gastroenterol. 2019;10:93-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 97. | Hoff G, Dominitz JA. Contrasting US and European approaches to colorectal cancer screening: which is best? Gut. 2010;59:407-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 98. | Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, Chen H, Dai M. Incidence, mortality, survival, risk factor and screening of colorectal cancer: A comparison among China, Europe, and northern America. Cancer Lett. 2021;522:255-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 126] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 99. | Ponti A, Anttila A, Ronco G, Senore C. Cancer screening in the European union, 2017 [Accessed 19 August 2021]. Available from: https://ec.europa.eu/health/sites/default/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf/. [Cited in This Article: ] |
| 100. | Altobelli E, D'Aloisio F, Angeletti PM. Colorectal cancer screening in countries of European Council outside of the EU-28. World J Gastroenterol. 2016;22:4946-4957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 45] [Cited by in F6Publishing: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 101. | Sung JJ, Ng SC, Chan FK, Chiu HM, Kim HS, Matsuda T, Ng SS, Lau JY, Zheng S, Adler S, Reddy N, Yeoh KG, Tsoi KK, Ching JY, Kuipers EJ, Rabeneck L, Young GP, Steele RJ, Lieberman D, Goh KL; Asia Pacific Working Group. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2015;64:121-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 102. | Sano Y, Byeon JS, Li XB, Wong MC, Chiu HM, Rerknimitr R, Utsumi T, Hattori S, Sano W, Iwatate M, Chiu P, Sung J. Colorectal cancer screening of the general population in East Asia. Dig Endosc. 2016;28:243-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 103. | Onyoh EF, Hsu WF, Chang LC, Lee YC, Wu MS, Chiu HM. The Rise of Colorectal Cancer in Asia: Epidemiology, Screening, and Management. Curr Gastroenterol Rep. 2019;21:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 104. | Ng SC, Wong SH. Colorectal cancer screening in Asia. Br Med Bull. 2013;105:29-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 105. | Loh KW, Majid HA, Dahlui M, Roslani AC, Su TT. Sociodemographic predictors of recall and recognition of colorectal cancer symptoms and anticipated delay in help- seeking in a multiethnic Asian population. Asian Pac J Cancer Prev. 2013;14:3799-3804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 106. | Shapiro JA, Bobo JK, Church TR, Rex DK, Chovnick G, Thompson TD, Zauber AG, Lieberman D, Levin TR, Joseph DA, Nadel MR. A Comparison of Fecal Immunochemical and High-Sensitivity Guaiac Tests for Colorectal Cancer Screening. Am J Gastroenterol. 2017;112:1728-1735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 107. | Sarakarn P, Promthet S, Vatanasapt P, Tipsunthonsak N, Jenwitheesuk K, Maneenin N, Jirapornkul C, Kamsa-ard S, Haengsorn T, Arkkhaboot C, Chen SL, Yen AM, Chiu SY, Fann JC, Chen TH. Preliminary Results: Colorectal Cancer Screening Using Fecal Immunochemical Test (FIT) in a Thai Population Aged 45-74 Years: A Population-Based Randomized Controlled Trial. Asian Pac J Cancer Prev. 2017;18:2883-2889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 108. | Ricciardiello L, Ferrari C, Cameletti M, Gaianill F, Buttitta F, Bazzoli F, Luigi de'Angelis G, Malesci A, Laghi L. Impact of SARS-CoV-2 Pandemic on Colorectal Cancer Screening Delay: Effect on Stage Shift and Increased Mortality. Clin Gastroenterol Hepatol. 2021;19:1410-1417.e9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 109. | Zorzi M, Hassan C, Capodaglio G, Baracco M, Antonelli G, Bovo E, Rugge M. Colonoscopy later than 270 days in a fecal immunochemical test-based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy. 2020;52:871-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 110. | Lee YC, Fann JC, Chiang TH, Chuang SL, Chen SL, Chiu HM, Yen AM, Chiu SY, Hsu CY, Hsu WF, Wu MS, Chen HH. Time to Colonoscopy and Risk of Colorectal Cancer in Patients With Positive Results From Fecal Immunochemical Tests. Clin Gastroenterol Hepatol. 2019;17:1332-1340.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 111. | Kadakuntla A, Wang T, Medgyesy K, Rrapi E, Litynski J, Adynski G, Tadros M. Colorectal cancer screening in the COVID-19 era. World J Gastrointest Oncol. 2021;13:238-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 21] [Cited by in F6Publishing: 26] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 112. | Akbulut S, Hargura AS, Garzali IU, Aloun A, Colak C. Clinical presentation, management, screening and surveillance for colorectal cancer during the COVID-19 pandemic. World J Clin Cases. 2022;10:9228-9240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Hasan MN, Haider N, Stigler FL, Khan RA, McCoy D, Zumla A, Kock RA, Uddin MJ. The Global Case-Fatality Rate of COVID-19 Has Been Declining Since May 2020. Am J Trop Med Hyg. 2021;104:2176-2184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 114. | Dadras O, Alinaghi SAS, Karimi A, MohsseniPour M, Barzegary A, Vahedi F, Pashaei Z, Mirzapour P, Fakhfouri A, Zargari G, Saeidi S, Mojdeganlou H, Badri H, Qaderi K, Behnezhad F, Mehraeen E. Effects of COVID-19 prevention procedures on other common infections: a systematic review. Eur J Med Res. 2021;26:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 115. | Cirrincione L, Plescia F, Ledda C, Rapisarda V, Martorana D, Moldovan RE, Theodoridou K, Cannizzaro E. COVID-19 pandemic: Prevention and protection measures to be adopted at the workplace. Sustainability. 2020;12:3603. [Cited in This Article: ] |
| 116. | Argulian E. Anticipating the "Second Wave" of Health Care Strain in the COVID-19 Pandemic. JACC Case Rep. 2020;2:845-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Gupta S, Lieberman D. Screening and Surveillance Colonoscopy and COVID-19: Avoiding More Casualties. Gastroenterology. 2020;159:1205-1208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 118. | Meijer J, Elferink MAG, van Hoeve JC, Buijsen J, van Erning F, Nagtegaal ID, Tanis PJ, Vink GR, Wumkes ML, de Hingh IHJT, Siesling S; On-behalf-of-the-COVID-and-Cancer-NL Consortium. Impact of the COVID-19 Pandemic on Colorectal Cancer Care in the Netherlands: A Population-based Study. Clin Colorectal Cancer. 2022;21:e171-e178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 119. | Shinkwin M, Silva L, Vogel I, Reeves N, Cornish J, Horwood J, Davies MM, Torkington J, Ansell J. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Dis. 2021;23:2014-2019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 120. | Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 121. | Aitken M, Kleinrock M. Shifts in Healthcare Demand, Delivery and Care During the COVID-19 Era: Tracking the Impact in the United States. IQVIA, 2020. [Cited in This Article: ] |
| 122. | Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, Rachet B, Aggarwal A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1142] [Cited by in F6Publishing: 1019] [Article Influence: 254.8] [Reference Citation Analysis (0)] |
| 123. | Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, Wu K, Cao Y, Ng K, Ogino S. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18:230-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 241] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 124. | Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, Siena S. Early-onset colorectal cancer in young individuals. Mol Oncol. 2019;13:109-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 125. | Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179-2185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 417] [Cited by in F6Publishing: 376] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 126. | Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 745] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 127. | Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, Zadnik V, Pellisé M, Esteban L, Kaminski MF, Suchanek S, Ngo O, Májek O, Leja M, Kuipers EJ, Spaander MC. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820-1826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 409] [Article Influence: 81.8] [Reference Citation Analysis (1)] |
| 128. | Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, Cantor SB, Chang GJ. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 650] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 129. | Willauer AN, Liu Y, Pereira AAL, Lam M, Morris JS, Raghav KPS, Morris VK, Menter D, Broaddus R, Meric-Bernstam F, Hayes-Jordan A, Huh W, Overman MJ, Kopetz S, Loree JM. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125:2002-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 130. | Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;122:929-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 131. | You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172:287-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 192] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 132. | REACCT Collaborative, Zaborowski AM, Abdile A, Adamina M, Aigner F, d'Allens L, Allmer C, Álvarez A, Anula R, Andric M, Atallah S, Bach S, Bala M, Barussaud M, Bausys A, Bebington B, Beggs A, Bellolio F, Bennett MR, Berdinskikh A, Bevan V, Biondo S, Bislenghi G, Bludau M, Boutall A, Brouwer N, Brown C, Bruns C, Buchanan DD, Buchwald P, Burger JWA, Burlov N, Campanelli M, Capdepont M, Carvello M, Chew HH, Christoforidis D, Clark D, Climent M, Cologne KG, Contreras T, Croner R, Daniels IR, Dapri G, Davies J, Delrio P, Denost Q, Deutsch M, Dias A, D'Hoore A, Drozdov E, Duek D, Dunlop M, Dziki A, Edmundson A, Efetov S, El-Hussuna A, Elliot B, Emile S, Espin E, Evans M, Faes S, Faiz O, Fleming F, Foppa C, Fowler G, Frasson M, Figueiredo N, Forgan T, Frizelle F, Gadaev S, Gellona J, Glyn T, Gong J, Goran B, Greenwood E, Guren MG, Guillon S, Gutlic I, Hahnloser D, Hampel H, Hanly A, Hasegawa H, Iversen LH, Hill A, Hill J, Hoch J, Hoffmeister M, Hompes R, Hurtado L, Iaquinandi F, Imbrasaite U, Islam R, Jafari MD, Kanemitsu Y, Karachun A, Karimuddin AA, Keller DS, Kelly J, Kennelly R, Khrykov G, Kocian P, Koh C, Kok N, Knight KA, Knol J, Kontovounisios C, Korner H, Krivokapic Z, Kronberger I, Kroon HM, Kryzauskas M, Kural S, Kusters M, Lakkis Z, Lankov T, Larson D, Lázár G, Lee KY, Lee SH, Lefèvre JH, Lepisto A, Lieu C, Loi L, Lynch C, Maillou-Martinaud H, Maroli A, Martin S, Martling A, Matzel KE, Mayol J, McDermott F, Meurette G, Millan M, Mitteregger M, Moiseenko A, Monson JRT, Morarasu S, Moritani K, Möslein G, Munini M, Nahas C, Nahas S, Negoi I, Novikova A, Ocares M, Okabayashi K, Olkina A, Oñate-Ocaña L, Otero J, Ozen C, Pace U, São Julião GP, Panaiotti L, Panis Y, Papamichael D, Park J, Patel S, Patrón Uriburu JC, Pera M, Perez RO, Petrov A, Pfeffer F, Phang PT, Poskus T, Pringle H, Proud D, Raguz I, Rama N, Rasheed S, Raval MJ, Rega D, Reissfelder C, Reyes Meneses JC, Ris F, Riss S, Rodriguez-Zentner H, Roxburgh CS, Saklani A, Salido AJ, Sammour T, Saraste D, Schneider M, Seishima R, Sekulic A, Seppala T, Sheahan K, Shine R, Shlomina A, Sica GS, Singnomklao T, Siragusa L, Smart N, Solis A, Spinelli A, Staiger RD, Stamos MJ, Steele S, Sunderland M, Tan KK, Tanis PJ, Tekkis P, Teklay B, Tengku S, Jiménez-Toscano M, Tsarkov P, Turina M, Ulrich A, Vailati BB, van Harten M, Verhoef C, Warrier S, Wexner S, de Wilt H, Weinberg BA, Wells C, Wolthuis A, Xynos E, You N, Zakharenko A, Zeballos J, Winter DC. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg. 2021;156:865-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 96] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 133. | Patel SG, May FP, Anderson JC, Burke CA, Dominitz JA, Gross SA, Jacobson BC, Shaukat A, Robertson DJ. Updates on Age to Start and Stop Colorectal Cancer Screening: Recommendations From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022;162:285-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 78] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 134. | Stoffel EM, Murphy CC. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology. 2020;158:341-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 254] [Article Influence: 63.5] [Reference Citation Analysis (1)] |
| 135. | Liang PS, Allison J, Ladabaum U, Martinez ME, Murphy CC, Schoen RE, Shaukat A, Tinmouth J, Gupta S. Potential Intended and Unintended Consequences of Recommending Initiation of Colorectal Cancer Screening at Age 45 Years. Gastroenterology. 2018;155:950-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 136. | Ladabaum U. Cost-Effectiveness of Current Colorectal Cancer Screening Tests. Gastrointest Endosc Clin N Am. 2020;30:479-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |